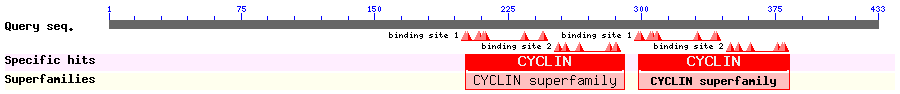
Summary for Cyclin B1 (NES ID: 12)
Full Name
G2/MITOTIC-SPECIFIC CYCLIN B1 UniProt
Alternative Names
None
Organism
Homo sapiens (Human)
Experimental Evidence for CRM1-mediated Export
LMB Sensitive, Binds CRM1 Ref.1Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint, Toyoshima et al., EMBO J, 1998 Ref.3Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1, Yang et al., Genes Dev, 1998 Ref.4Using a Simple Cellular Assay to Map NES Motifs in Cancer-Related Proteins, Gain Insight into CRM1-Mediated NES Export, and Search for NES-Harboring Micropeptides, Sendino et al., Int J Mol Sci, 2020
Mutations That Affect Nuclear Export
*highlighted yellow in the full sequence
V149A/L151A/V153A Ref.1Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint, Toyoshima et al., EMBO J, 1998 Ref.2MPF localization is controlled by nuclear export, Hagting et al., EMBO J, 1998 Mutations That Affect CRM1 Binding
*shown as red residues in the full sequence
F146A Ref.3Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1, Yang et al., Genes Dev, 1998 Functional Export Signals
*shown as underlined residues in the full sequence
141DLCQAFSDVILA152 Ref.1Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint, Toyoshima et al., EMBO J, 1998 Secondary Structure of Export Signal
Unknown
Other Residues Important for Export
Unknown
Sequence
Show FASTA Format
Show Domain Info by CDD
Show Secondary Structure by PSIPRED
Show Conservation Score by AL2CO
10 20 30 40 50 60MALRVTRNSK INAENKAKIN MAGAKRVPTA PAATSKPGLR PRTALGDIGN KVSEQLQAKM70 80 90 100 110 120PMKKEAKPSA TGKVIDKKLP KPLEKVPMLV PVPVSEPVPE PEPEPEPEPV KEEKLSPEPI130 140 150 160 170 180LVDTASPSPM ETSGCAPAEE DLCQAFSDVI LAVNDVDAED GADPNLCSEY VKDIYAYLRQ190 200 210 220 230 240LEEEQAVRPK YLLGREVTGN MRAILIDWLV QVQMKFRLLQ ETMYMTVSII DRFMQNNCVP250 260 270 280 290 300KKMLQLVGVT AMFIASKYEE MYPPEIGDFA FVTDNTYTKH QIRQMEMKIL RALNFGLGRP310 320 330 340 350 360LPLHFLRRAS KIGEVDVEQH TLAKYLMELT MLDYDMVHFP PSQIAAGAFC LALKILDNGE370 380 390 400 410 420WTPTLQHYLS YTEESLLPVM QHLAKNVVMV NQGLTKHMTV KNKYATSKHA KISTLPQLNS430ALVQDLAKAV AKV
3D Structures in PDB
6GU2 ( X-ray ,2.00 Å resolution)
Comments
Cyclin B1/Cdc2 complex is a key regulator of cell cycle transition. Cyclin B1 accumulates in the cytoplasm during S and G2 phase and moves to the nucleus before nuclear envelope breakdown. It was previously found that there is a domain of Cyclin B1 (called CRS) in human that was sufficient for its cytoplasmic localization. Toyoshima et al. demonstrated that aa 141-152 can export NES-OVA conjugate. The CRS domain and NES function are conserved in Xenoopus (UniProt: P13350) as shown by Yang et al. In addition, they indicated that CRS contains four serine phosphorylation sites, which are important for Cyclin B1 nuclear accumulation. Mutation of Ser to Glu, which mimics constitutive phosphorylation, reduces the affinity of Cyclin B1 for CRM1. Residue 138-156 was also tested by Sendino et al. in Rev1.4-GFP and SRVA/B reporters which responded to CRM1 co-expression. Ref.3Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1, Yang et al., Genes Dev, 1998 Ref.4Using a Simple Cellular Assay to Map NES Motifs in Cancer-Related Proteins, Gain Insight into CRM1-Mediated NES Export, and Search for NES-Harboring Micropeptides, Sendino et al., Int J Mol Sci, 2020
References
[1]. "Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint"
Toyoshima, F., Moriguchi, T., Wada, A., Fukuda, M., Nishida, E. (1998) EMBO J, 17:2728-2735 PubMed
[2]. "MPF localization is controlled by nuclear export"
Hagting A, Karlsson C, Clute P, Jackman M, Pines J. (1998) EMBO J, 14:4127-38 PubMed
[3]. "Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1"
Yang, J., Bardes, E.S., Moore, J.D., Brennan, J., Powers, M.A., Kornbluth, S. (1998) Genes Dev, 12:2131-2143 PubMed
[4]. "Using a Simple Cellular Assay to Map NES Motifs in Cancer-Related Proteins, Gain Insight into CRM1-Mediated NES Export, and Search for NES-Harboring Micropeptides"
Sendino M, Omaetxebarria MJ, Prieto G, Rodriguez JA. (2020) Int J Mol Sci, 21(17):E6341 PubMed
Toyoshima, F., Moriguchi, T., Wada, A., Fukuda, M., Nishida, E. (1998) EMBO J, 17:2728-2735 PubMed
[2]. "MPF localization is controlled by nuclear export"
Hagting A, Karlsson C, Clute P, Jackman M, Pines J. (1998) EMBO J, 14:4127-38 PubMed
[3]. "Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1"
Yang, J., Bardes, E.S., Moore, J.D., Brennan, J., Powers, M.A., Kornbluth, S. (1998) Genes Dev, 12:2131-2143 PubMed
[4]. "Using a Simple Cellular Assay to Map NES Motifs in Cancer-Related Proteins, Gain Insight into CRM1-Mediated NES Export, and Search for NES-Harboring Micropeptides"
Sendino M, Omaetxebarria MJ, Prieto G, Rodriguez JA. (2020) Int J Mol Sci, 21(17):E6341 PubMed
User Input
Accurate identification of NESs is difficult because many sequences in the genome match the NES consensus.
Therefore, some published NESs may be mistakenly identified. Please help us improve the accuracy of NESdb
by providing either a positive or negative flag for the NES in this entry. Supporting comments are required to process the flag.