Estrogen Receptor, ERα Estradiol receptor, Nuclear receptor subfamily 3 group A member 1
Show FASTA Format
>gi|544257|sp|P03372.2|ESR1_HUMAN RecName: Full=Estrogen receptor; Short=ER; AltName: Full=ER-alpha; AltName: Full=Estradiol receptor; AltName: Full=Nuclear receptor subfamily 3 group A member 1
MTMTLHTKASGMALLHQIQGNELEPLNRPQLKIPLERPLGEVYLDSSKPAVYNYPEGAAYEFNAAAAANA
QVYGQTGLPYGPGSEAAAFGSNGLGGFPPLNSVSPSPLMLLHPPPQLSPFLQPHGQQVPYYLENEPSGYT
VREAGPPAFYRPNSDNRRQGGRERLASTNDKGSMAMESAKETRYCAVCNDYASGYHYGVWSCEGCKAFFK
RSIQGHNDYMCPATNQCTIDKNRRKSCQACRLRKCYEVGMMKGGIRKDRRGGRMLKHKRQRDDGEGRGEV
GSAGDMRAANLWPSPLMIKRSKKNSLALSLTADQMVSALLDAEPPILYSEYDPTRPFSEASMMGLLTNLA
DRELVHMINWAKRVPGFVDLTLHDQVHLLECAWLEILMIGLVWRSMEHPGKLLFAPNLLLDRNQGKCVEG
MVEIFDMLLATSSRFRMMNLQGEEFVCLKSIILLNSGVYTFLSSTLKSLEEKDHIHRVLDKITDTLIHLM
AKAGLTLQQQHQRLAQLLLILSHIRHMSNKGMEHLYSMKCKNVVPLYDLLLEMLDAHRLHAPTSRGGASV
EETDQSHLATAGSTSSHSLQKYYITGEAEGFPATV
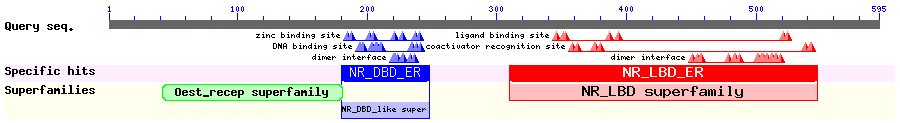
Show Domain Info by CDD
Show Secondary Structure by PSIPRED
# PSIPRED HFORMAT (PSIPRED V3.2)
Conf: 975656444665201331147766558998778877888875778999940269999634
Pred: CCCCCCCCCCCCCCHHHHCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
AA: MTMTLHTKASGMALLHQIQGNELEPLNRPQLKIPLERPLGEVYLDSSKPAVYNYPEGAAY
10 20 30 40 50 60
Conf: 455532357888889999999999654578999999999997899985323899999998
Pred: CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
AA: EFNAAAAANAQVYGQTGLPYGPGSEAAAFGSNGLGGFPPLNSVSPSPLMLLHPPPQLSPF
70 80 90 100 110 120
Conf: 789999887666789998743478998877899964223564434678865542111344
Pred: CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
AA: LQPHGQQVPYYLENEPSGYTVREAGPPAFYRPNSDNRRQGGRERLASTNDKGSMAMESAK
130 140 150 160 170 180
Conf: 423877740323677654223033478886420178876576874221000000002223
Pred: CCEEEEEECCCCCCCCCCCCCHHHHHHHHHHHHCCCCCCCCCCCCCCCCCHHHHHHHHHH
AA: ETRYCAVCNDYASGYHYGVWSCEGCKAFFKRSIQGHNDYMCPATNQCTIDKNRRKSCQAC
190 200 210 220 230 240
Conf: 103211013002543456679876676558999999987568876666767999632246
Pred: HCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
AA: RLRKCYEVGMMKGGIRKDRRGGRMLKHKRQRDDGEGRGEVGSAGDMRAANLWPSPLMIKR
250 260 270 280 290 300
Conf: 555545668999999999862299922144599999628789997311011458888774
Pred: CCCCCCCCCCCHHHHHHHHHHCCCCCEECCCCCCCCCHHHHHHHHHHCCHHHHHHHHHHH
AA: SKKNSLALSLTADQMVSALLDAEPPILYSEYDPTRPFSEASMMGLLTNLADRELVHMINW
310 320 330 340 350 360
Conf: 102886322470012244424568999987856426689918622651013786532101
Pred: HCCCCCCCCCCCHHHHHHHHHHHHHHHHHHHHHHCCCCCCCEEECCCCCCCCCCCCCCCH
AA: AKRVPGFVDLTLHDQVHLLECAWLEILMIGLVWRSMEHPGKLLFAPNLLLDRNQGKCVEG
370 380 390 400 410 420
Conf: 999999999998876640455311345566667449952234343213234889999999
Pred: HHHHHHHHHHHHHHHHHHCCCCCHHHHHHHHHHHCCCCCCCCCCCCCCHHHHHHHHHHHH
AA: MVEIFDMLLATSSRFRMMNLQGEEFVCLKSIILLNSGVYTFLSSTLKSLEEKDHIHRVLD
430 440 450 460 470 480
Conf: 899999999998199912455469899999766664434678886514368811247778
Pred: HHHHHHHHHHHHCCCCCHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHCCCCCCCCHHHHH
AA: KITDTLIHLMAKAGLTLQQQHQRLAQLLLILSHIRHMSNKGMEHLYSMKCKNVVPLYDLL
490 500 510 520 530 540
Conf: 9898201025888899999865546787889999998988632378788999999
Pred: HHHHHCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC
AA: LEMLDAHRLHAPTSRGGASVEETDQSHLATAGSTSSHSLQKYYITGEAEGFPATV
550 560 570 580 590
Show Conservation Score by AL2CO
1 M -1.000 *
2 T -1.000 *
3 M -1.000 *
4 T -1.000 *
5 L -1.000 *
6 H -1.000 *
7 T -1.000 *
8 K -1.000 *
9 A -1.000 *
10 S -1.000 *
11 G -1.000 *
12 M -1.000 *
13 A -1.000 *
14 L -1.000 *
15 L -1.000 *
16 H -1.000 *
17 Q -1.000 *
18 I -1.000 *
19 Q -1.000 *
20 G -1.000 *
21 N -1.000 *
22 E -1.000 *
23 L -1.000 *
24 E -1.000 *
25 P -1.000 *
26 L -1.000 *
27 N -1.000 *
28 R -1.000 *
29 P -1.000 *
30 Q -1.000 *
31 L -1.000 *
32 K -1.000 *
33 I -1.000 *
34 P -1.000 *
35 L -1.000 *
36 E -1.000 *
37 R -1.000 *
38 P -1.000 *
39 L -1.000 *
40 G -1.000 *
41 E -1.000 *
42 V -1.000 *
43 Y -1.000 *
44 L -1.000 *
45 D -1.000 *
46 S -1.000 *
47 S -1.000 *
48 K -1.000 *
49 P -1.000 *
50 A -1.000 *
51 V -1.000 *
52 Y -1.000 *
53 N -1.000 *
54 Y -1.000 *
55 P -1.000 *
56 E -1.000 *
57 G -1.000 *
58 A -1.000 *
59 A -1.000 *
60 Y -1.000 *
61 E -0.543
62 F -0.721
63 N -0.633
64 A -0.784
65 A -0.495
66 A -0.861
67 A -0.805
68 A -0.863
69 N -0.767
70 A -0.670
71 Q -0.745
72 V -0.523
73 Y -0.756
74 G -0.414
75 Q -0.792
76 T -0.765
77 G -0.556
78 L -1.000 *
79 P -1.000 *
80 Y -0.752
81 G -0.749
82 P -1.000 *
83 G -1.000 *
84 S -1.000 *
85 E -0.861
86 A -0.933
87 A -0.984
88 A -0.549
89 F -0.776
90 G -0.756
91 S -0.886
92 N -0.691
93 G -0.598
94 L -0.897
95 G -0.791
96 G -0.878
97 F -0.982
98 P -0.904
99 P -0.767
100 L -0.946
101 N -0.961
102 S -0.732
103 V -0.863
104 S -0.740
105 P -0.576
106 S -0.343
107 P -0.642
108 L -0.878
109 M -0.969
110 L -0.750
111 L -0.770
112 H -0.898
113 P -0.607
114 P -0.751
115 P -0.883
116 Q -0.663
117 L -0.851
118 S -0.667
119 P -0.235
120 F -0.772
121 L -0.775
122 Q -0.908
123 P -0.833
124 H -0.993
125 G -0.956
126 Q -0.805
127 Q -0.918
128 V -0.841
129 P -0.798
130 Y -0.879
131 Y -1.024
132 L -0.985
133 E -0.879
134 N -0.848
135 E -0.858
136 P -0.950
137 S -0.894
138 G -0.900
139 Y -1.096
140 T -0.995
141 V -0.974
142 R -0.976
143 E -0.921
144 A -0.849
145 G -0.860
146 P -0.843
147 P -0.881
148 A -1.008
149 F -0.985
150 Y -1.092
151 R -0.984
152 P -0.652
153 N -0.995
154 S -0.862
155 D -0.895
156 N -0.918
157 R -0.881
158 R -1.005
159 Q -1.024
160 G -0.951
161 G -0.904
162 R -0.911
163 E -0.955
164 R -0.900
165 L -0.963
166 A -0.964
167 S -0.811
168 T -0.979
169 N -0.794
170 D -0.901
171 K -0.924
172 G -0.903
173 S -0.880
174 M -0.911
175 A -0.828
176 M -0.960
177 E -0.654
178 S -0.782
179 A -0.841
180 K -0.749
181 E -0.370
182 T -0.645
183 R 0.026
184 Y -0.038
185 C 2.305
186 A 0.249
187 V 1.700
188 C 2.310
189 N 0.374
190 D 1.867
191 Y -0.071
192 A 0.842
193 S 0.954
194 G 2.045
195 Y 0.782
196 H 2.062
197 Y 2.305
198 G 1.426
199 V 1.657
200 W 0.394
201 S 1.305
202 C 3.559
203 E 2.245
204 G 1.750
205 C 2.743
206 K 2.133
207 A 1.660
208 F 2.409
209 F 2.346
210 K 1.696
211 R 1.872
212 S 2.126
213 I 1.555
214 Q 0.793
215 G 1.181
216 H -0.020
217 N -0.170
218 D -0.545
219 Y 1.995
220 M -0.188
221 C 3.074
222 P 0.497
223 A -0.126
224 T -0.117
225 N 0.513
226 Q -0.189
227 C 2.387
228 T -0.154
229 I 1.333
230 D 1.287
231 K 0.748
232 N -0.016
233 R 0.489
234 R 2.481
235 K 1.328
236 S 0.588
237 C 2.020
238 Q 1.749
239 A 0.355
240 C 2.020
241 R 1.964
242 L 1.277
243 R 0.137
244 K 1.503
245 C 2.517
246 Y -0.029
247 E -0.223
248 V 0.416
249 G 2.418
250 M 1.623
251 M -0.145
252 K 0.397
253 G 0.584
254 G 0.224
255 I -0.245
256 R 0.125
257 K -0.421
258 D 0.064
259 R 0.134
260 R -0.744
261 G -0.786
262 G -0.866
263 R -0.610
264 M -0.931
265 L -0.890
266 K -0.502
267 H -0.978
268 K -0.645
269 R -0.650
270 Q -0.835
271 R -0.850
272 D -0.568
273 D -0.836
274 G -0.982
275 E -1.041
276 G -0.791
277 R -0.974
278 G -0.980
279 E -0.782
280 V -0.923
281 G -0.830
282 S -0.960
283 A -0.846
284 G -0.942
285 D -0.724
286 M -0.924
287 R -0.868
288 A -0.994
289 A -0.958
290 N -0.721
291 L -1.013
292 W -1.045
293 P -0.823
294 S -0.855
295 P -0.937
296 L -0.982
297 M -0.974
298 I -1.020
299 K -1.013
300 R -1.051
301 S -0.869
302 K -0.975
303 K -1.008
304 N -1.041
305 S -0.864
306 L -0.800
307 A -1.015
308 L -0.957
309 S -0.959
310 L -0.663
311 T -0.906
312 A -0.953
313 D -0.356
314 Q -0.624
315 M -0.690
316 V -0.157
317 S -0.858
318 A -0.863
319 L -0.746
320 L -0.733
321 D -0.864
322 A -0.860
323 E -0.540
324 P -0.723
325 P -0.581
326 I -1.010
327 L -0.773
328 Y -0.955
329 S -0.809
330 E -1.045
331 Y -0.746
332 D -0.820
333 P -0.669
334 T -1.028
335 R -0.936
336 P -0.546
337 F -0.798
338 S -0.507
339 E -0.868
340 A -0.558
341 S -0.507
342 M -0.553
343 M -0.557
344 G -0.311
345 L -0.388
346 L 0.611
347 T 0.136
348 N -0.044
349 L 0.323
350 A 0.091
351 D 0.077
352 R -0.161
353 E 0.319
354 L 1.297
355 V 0.109
356 H -0.514
357 M 0.217
358 I 1.438
359 N 0.102
360 W 1.353
361 A 2.088
362 K 1.526
363 R 0.043
364 V 1.081
365 P 2.005
366 G 0.426
367 F 1.575
368 V -0.549
369 D -0.063
370 L 1.316
371 T -0.365
372 L 0.447
373 H -0.673
374 D 1.237
375 Q 1.836
376 V 0.867
377 H -0.389
378 L 1.772
379 L 2.273
380 E 0.773
381 C 0.290
382 A 0.505
383 W 1.227
384 L 0.619
385 E 2.200
386 I 0.955
387 L 1.166
388 M 0.785
389 I 0.351
390 G 0.355
391 L 0.469
392 V 0.783
393 W 0.264
394 R 2.073
395 S 2.730
396 M 0.162
397 E -0.489
398 H -0.699
399 P -0.206
400 G 0.407
401 K -0.629
402 L 1.015
403 L -0.257
404 F 1.512
405 A 0.313
406 P -0.374
407 N 1.056
408 L 0.176
409 L -0.412
410 L 0.466
411 D 0.019
412 R 0.073
413 N -0.390
414 Q -0.301
415 G -0.052
416 K -0.453
417 C -0.358
418 V -0.453
419 E -0.478
420 G -0.080
421 M -0.186
422 V -0.689
423 E 0.214
424 I 0.332
425 F -0.200
426 D 0.008
427 M -0.493
428 L 0.115
429 L -0.072
430 A 0.207
431 T 0.402
432 S 0.540
433 S -0.147
434 R 0.329
435 F 1.012
436 R -0.146
437 M -0.677
438 M 1.086
439 N -0.389
440 L 1.269
441 Q 0.129
442 G -0.785
443 E 0.085
444 E 2.724
445 F 0.858
446 V 0.304
447 C 0.976
448 L 2.885
449 K 0.810
450 S 1.968
451 I 1.291
452 I 0.746
453 L 2.225
454 L 0.294
455 N 1.574
456 S 0.834
457 G 0.314
458 V -0.026
459 Y -0.162
460 T -0.101
461 F -0.240
462 L -0.259
463 S -1.000 *
464 S -0.385
465 T -0.332
466 L -0.427
467 K -0.487
468 S -0.429
469 L -0.285
470 E -0.526
471 E 0.174
472 K -0.735
473 D -0.730
474 H -0.563
475 I 1.895
476 H -0.557
477 R -0.735
478 V 0.844
479 L 1.268
480 D 0.204
481 K -0.763
482 I 0.742
483 T 0.118
484 D -0.125
485 T 0.816
486 L 2.685
487 I -0.422
488 H -0.467
489 L -0.084
490 M 0.127
491 A -0.459
492 K -0.141
493 A 0.018
494 G -0.087
495 L -0.858
496 T -0.443
497 L -0.863
498 Q 0.194
499 Q -0.311
500 Q -0.052
501 H -0.655
502 Q -0.356
503 R 1.756
504 L 0.144
505 A 1.034
506 Q 0.560
507 L 1.843
508 L 1.271
509 L 0.899
510 I 0.317
511 L 2.589
512 S 1.300
513 H 0.241
514 I 1.431
515 R 1.129
516 H 0.846
517 M 0.788
518 S 0.701
519 N -0.142
520 K 0.412
521 G 0.261
522 M 0.289
523 E 0.849
524 H -0.152
525 L 0.287
526 Y -0.060
527 S -0.546
528 M 0.599
529 K 0.957
530 C -0.185
531 K -0.421
532 N 0.770
533 V -0.570
534 V 0.190
535 P 0.190
536 L 0.736
537 Y 0.354
538 D 0.118
539 L 1.634
540 L 1.434
541 L -0.164
542 E 1.210
543 M 1.180
544 L 1.628
545 D 0.853
546 A 0.837
547 H 0.461
548 R -0.525
549 L -0.470
550 H -1.000 *
551 A -1.000 *
552 P -1.000 *
553 T -1.000 *
554 S -1.000 *
555 R -1.000 *
556 G -1.000 *
557 G -1.000 *
558 A -1.000 *
559 S -1.000 *
560 V -1.000 *
561 E -1.000 *
562 E -1.000 *
563 T -1.000 *
564 D -1.000 *
565 Q -1.000 *
566 S -1.000 *
567 H -1.000 *
568 L -1.000 *
569 A -1.000 *
570 T -1.000 *
571 A -1.000 *
572 G -1.000 *
573 S -1.000 *
574 T -1.000 *
575 S -1.000 *
576 S -1.000 *
577 H -1.000 *
578 S -1.000 *
579 L -1.000 *
580 Q -1.000 *
581 K -1.000 *
582 Y -1.000 *
583 Y -1.000 *
584 I -1.000 *
585 T -1.000 *
586 G -1.000 *
587 E -1.000 *
588 A -1.000 *
589 E -1.000 *
590 G -1.000 *
591 F -1.000 *
592 P -1.000 *
593 A -1.000 *
594 T -1.000 *
595 V -1.000 *
* gap fraction no less than 0.50; conservation set to M-S
M: mean; S: standard deviation
al2co - The parameters are:
Input alignment file - 166.paln
Output conservation - STDOUT
Weighting scheme - independent-count based
Conservation calculation method - entropy-based
Window size - 1
Conservation normalized to zero mean and unity variance
Gap fraction to suppress calculation - 0.50
10 20 30 40 50 60
MTMTLHTKAS GMALLHQIQG NELEPLNRPQ LKIPLERPLG EVYLDSSKPA VYNYPEGAAY
70 80 90 100 110 120
EFNAAAAANA QVYGQTGLPY GPGSEAAAFG SNGLGGFPPL NSVSPSPLML LHPPPQLSPF
130 140 150 160 170 180
LQPHGQQVPY YLENEPSGYT VREAGPPAFY RPNSDNRRQG GRERLASTND KGSMAMESAK
190 200 210 220 230 240
ETRYCAVCND YASGYHYGVW SCEGCKAFFK RSIQGHNDYM CPATNQCTID KNRRKSCQAC
250 260 270 280 290 300
RLRKCYEVGM MKGGIRKDRR GGRMLKHKRQ RDDGEGRGEV GSAGDMRAAN LWPSPLMIKR
310 320 330 340 350 360
SKKNSLALSL TADQMVSALL DAEPPILYSE YDPTRPFSEA SMMGLLTNLA DRELVHMINW
370 380 390 400 410 420
AKRVPGFVDL TLHDQVHLLE CAWLEILMIG LVWRSMEHPG KLLFAPNLLL DRNQGKCVEG
430 440 450 460 470 480
MVEIFDMLLA TSSRFRMMNL QGEEFVCLKS IILLNSGVYT FLSSTLKSLE EKDHIHRVLD
490 500 510 520 530 540
KITDTLIHLM AKAGLTLQQQ HQRLAQLLLI LSHIRHMSNK GMEHLYSMKC KNVVPLYDLL
550 560 570 580 590
LEMLDAHRLH APTSRGGASV EETDQSHLAT AGSTSSHSLQ KYYITGEAEG FPATV
2OCF (X-Ray,2.95 Å resolution)
Lombardi et al. reported that ERα nuclear export is dependent on estradiol-mediated phosphatidylinositol-3-kinase (PI3K)/AKT activation and LMB sensitive. CRM1 can be found in co-IP with ERα. A putative NES was identified, which is active upon actinomycin D treatment and can be inhibited by LMB. Peptide containing putative NES can inhibit CRM1 interaction and ERα nuclear export. Castoria et al. reported that Tyr537 phosphorylation is critical for estradiol-sensitive CRM1 interaction and nuclear export. Souza et al. more recently reported that both ERα/β are sensitive to LMB treatment. It is unclear whether ERβ contain putative NES. It should be noted that the proposed NES is in a folded structure.
Doubts about NES: The proposed NES failed to bind CRM1 in GST-pulldown assay (Chook Lab, unpublished results).
Ref.2Tyrosine phosphorylation of estradiol receptor by Src regulates its hormone-dependent nuclear export and cell cycle progression in breast cancer cells, Castoria et al., Oncogene, 2012 Ref.3Estrogen Receptors Localization and Signaling Pathways in DU-145 Human Prostate Cancer Cells, Souza et al., Mol Cell Endocrinol, 2019
[1]. "Hormone-dependent nuclear export of estradiol receptor and DNA synthesis in breast cancer cells."
Lombardi M, Castoria G, Migliaccio A, Barone MV, Di Stasio R, Ciociola A, Bottero D, Yamaguchi H, Appella E, Auricchio F. (2008)
J Cell Biol,
182:327-40
PubMed[2]. "Tyrosine phosphorylation of estradiol receptor by Src regulates its hormone-dependent nuclear export and cell cycle progression in breast cancer cells"
Castoria G, Giovannelli P, Lombardi M, De Rosa C, Giraldi T, de Falco A, Barone MV, Abbondanza C, Migliaccio A, Auricchio F. (2012)
Oncogene,
31(46):4868-77
PubMed[3]. "Estrogen Receptors Localization and Signaling Pathways in DU-145 Human Prostate Cancer Cells"
Souza DS, Lombardi APG, Vicente CM, Lucas TFG, Erustes AG, Pereira GJS, Porto CS. (2019)
Mol Cell Endocrinol,
483:11-23
PubMed
Accurate identification of NESs is difficult because many sequences in the genome match the NES consensus.
Therefore, some published NESs may be mistakenly identified. Please help us improve the accuracy of NESdb
by providing either a positive or negative flag for the NES in this entry. Supporting comments are required to process the flag.
