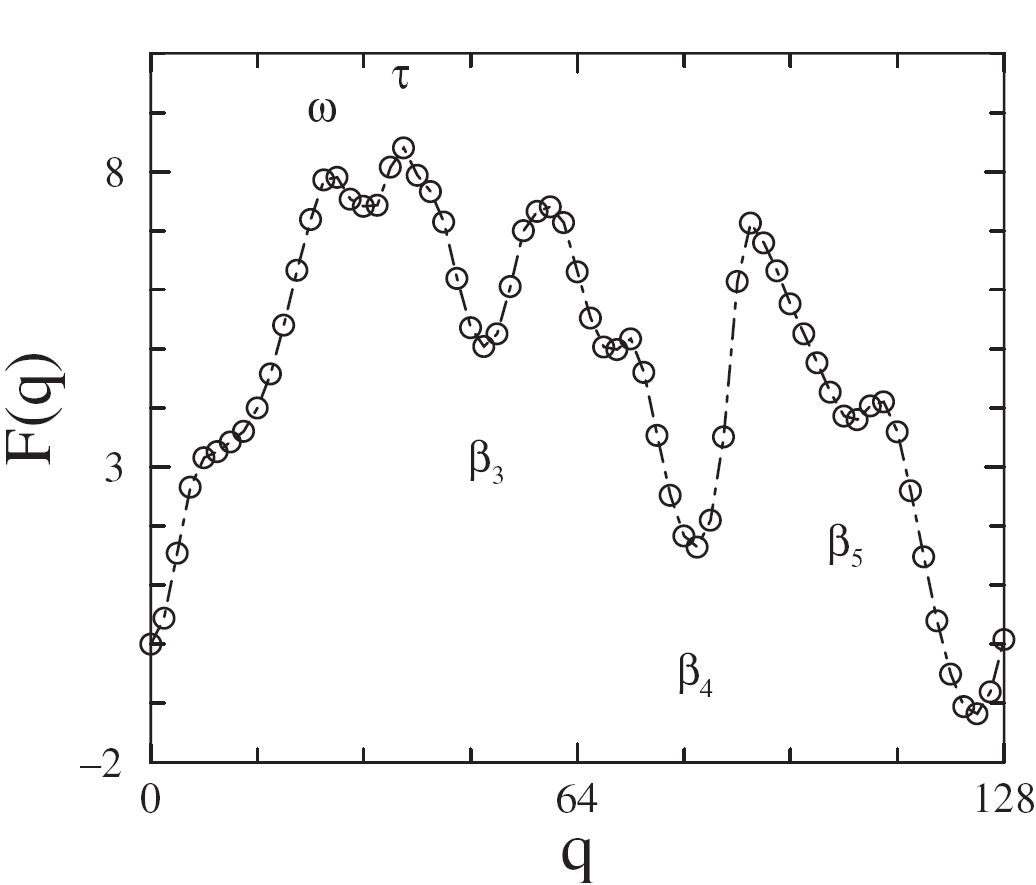
Structural events along the free energy profile F(q) of CheY: This simple model assumes that each residue is either folded (is in its native state) or unfolded. q is the number of folded residues. The misfolded helical intermediate is centered at q=24 (ω), and the nucleus, β1-α1-β2, is formed at q=38 (τ). Each major basin in the profile corresponds to the completion of a helix αn (left side of basin), formation of a strand βn+1 (middle of basin), and partial formation of the following helix αn+1 (right side of basin). The structure of the misfolded intermediate, and the registry of helices with maxima in F(q) indicates topological frustration between the β-interior and α-exterior of the protein. The depth of minima (height of maxima) in this region reflect loop closure events that are sensitive to the entropy approximations used in these types of models.