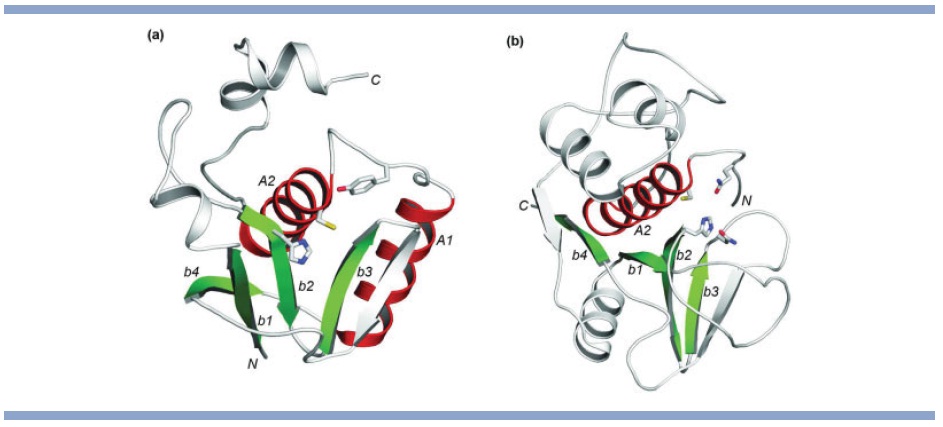
RID domains have papain-like fold. A Rho GTPase inactivation domain (RID) has been discovered in the multifunctional, autoprocessing RTX toxin RtxA from Vibrio cholerae. The RID domain causes actin depolymerization and rounding of host cells through inactivation of the small Rho GTPases Rho, Rac, and Cdc42. With only a few toxin proteins containing RID domains in the current sequence database, the structure and molecular mechanisms of this domain are unknown. Using comparative sequence and structural analyses, we report homology inference, fold recognition, and active site prediction for RID domains. Remote homologs of RID domains were identified in two other experimentally characterized bacterial virulence factors: IcsB of Shigella flexneri and BopA of Burkholderia pseudomallei, as well as in a group of uncharacterized bacterial membrane proteins. IcsB plays an important role in helping Shigella to evade the host autophagy defense system. RID domain homologs share a conserved diad of cysteine and histidine residues, and are predicted to adopt a circularly permuted papain-like thiol protease fold. RID domains from MARTX toxins and virulence factors IcsB and BopA thus could function as proteases or acyltransferases acting on host molecules. Our results provide structural and mechanistic insights into several important proteins functioning in bacterial pathogenesis. The above picture shows representative structures with (a) a circularly permuted papain-like fold (pdb: 3ebq) and (b) a papain-like fold (pdb: 1khq). a-Helices and b-strands in structural core regions are colored in red and green respectively. Other parts of the structures are colored in gray. Side chains of catalytically important residues are shown as sticks. N- and C- termini are marked.