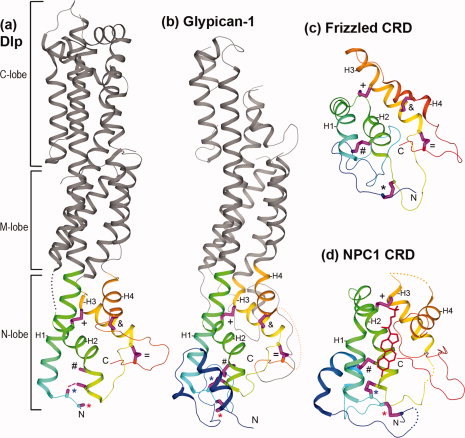
Structural diagrams of HFN-CRD and FZ-CRD domains. N- and C-termini are labeled for the CRDs. Four core alpha helices in these CRDs are labeled H1, H2, H3, and H4, respectively. Sidechains of conserved cysteines, most of which form disulfide bonds in the structures, are shown as sticks and colored magenta. They are labeled the same way as in Figure 1. (a) The glypican core region of Drosophila Dlp (PDB: 3odn). Three previously defined sub-domains of glypican are labeled as N-, M-, and C-lobes, respectively. The N-lobe has an HFN-CRD fold and is colored in rainbow from N- to C-terminus, while the M- and C-lobes, formed mainly by insertions from the N-lobe, are colored in grey. The N-terminal sequence segment before the CC motif in the C*C*CC*CX9CX2C*CX3CX6C*C*C pattern is disordered. Sidechains of the two cysteines in the CC motif (C3 and C4) are also partially disordered. Therefore, the inferred disulfide bond between C4 and C7 (labeled by a blue *) are shown as a dashed line. (b) The glypican core region of human Glypican-1 (PDB: 4acr). One disordered region in the N-lobe (between the last two conserved cysteines) is displayed by a dotted connection. (c) The spatial structure of FZ-CRD (PDB: 1ijy) from the mouse protein Frizzled8. (d) The spatial structure of an HFN-CRD (PDB: 3gkj) from human NPC1. To aid the view of conserved disulfide bonds, some insertions to the NPC1 CRD are replaced by dotted connections. The bound cholesterol molecule is shown in red line.